Abstract
Introduction: Dysregulated B-cell receptor (BCR) signaling drives proliferation of Waldenstrom macroglobulinemia (WM) cells, which characteristically secrete excessive IgM. To maintain IgM production and preserve cellular homeostasis, WM cells rely on optimally functioning proteasomes to degrade and recycle proteins. This biological dependence has been clinically exploited using the proteasome inhibitor (PI), bortezomib (Btz), which is capable of inducing remission in 85% of WM patients (Treon et al Clin Canc Res, 2007) as a single agent. Despite, its activity, Btz use is associated with a high incidence of sensory and autonomic neuropathies as well as its cumbersome dosing schedule and non-oral route of administration. Thus, new agents that 1.) build on the activity of Btz, 2.) have a more favorable toxicity profile and 3.) can be combined with standard of care agents such as, ibrutinib (Ibr), are highly attractive and should be prioritized for investigation. Here we describe the preclinical activity of ixazomib (Ixa), a PI that is administered orally and is associated with significantly less peripheral neuropathy; in combination with Ibr, in WM cells.
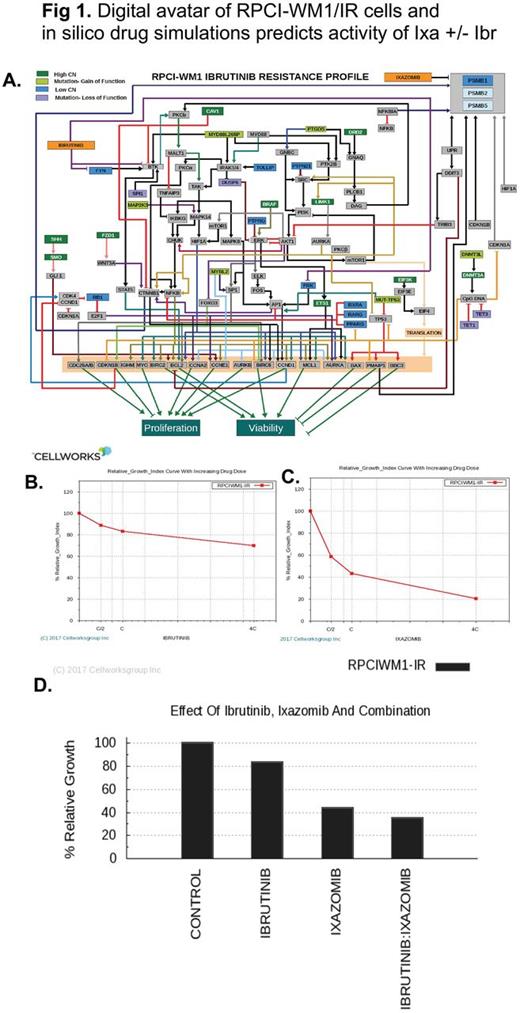
Methods: WM cell lines (BCWM.1 and RPCI.WM1) and their drug-resistant subclones were used. Genomic data including whole exome sequencing (WES) and copy number variations (CNV) was used in creation of digital simulation avatars of RPCI-WM1 and RPCI-WM1/IR (Ibr-resistant, Ibr-R variant), where the salient and prominently dysregulated cellular pathways were identified. Ixa +/- Ibr was tested in digital avatars and dose-dependent impact on simulated disease growth was assessed (Fig 1A). The predictive results were experimentally validated. Cell viability was measured using trypan blue or CellTiter Glo. Apoptosis was examined by annexin-V/PI staining of drug-treated cells followed by flow cytometry analysis. Mitochondrial membrane permeability (MOMP) was determined by TMRM staining of drug-treated cells followed by flow cytometry.
Results: We first profiled the growth inhibitory action of Ixa in WM cell lines (n=9); all of which were sensitive to Ixa irrespective of acquired resistance to Btz, Ibr or carfilzomib with a median IC50 of 95.59nM. We then tested whether Ixa could cooperate with Ibr to enhance WM cell kill. Indeed, the Cellworks modeling tool, predicted a significant loss of viability and induction of apoptosis in both wildtype and Ibr-R cells treated with Ibr + Ixa, as compared to either single agent (Fig 1B-D). We confirmed this in vitro, where viability of wildtype WM cells treated with Ixa (20nM), Ibr (5uM) or Ibr + Ixa for 6h significantly decreased; most notable with Ixa + Ibr (25.5% viable cells). And this effect was similar in Ibr-R cell lines (30.3% viable cells) after combination treatment. Further confirming in silico predictive modeling results, we noted a significant increase in annexin-V/PI positivity in Ixa treated wildtype (24.5%) and Ibr-R WM cell lines (33.1%). Whereas Ibr alone exhibited minimal apoptosis in WM cell lines (~11 - 15%), the combination of with Ixa + Ibr enhanced programmed cell death in wildtype (40.2%) and Ibr-R cells (50.5%). As intrinsic apoptosis is triggered through mitochondrial disruption, we also examined mitochondrial transmembrane permeability (MOMP) in cells treated with Ibr, Ixa or Ixa + Ibr. In wildtype cells, mean MOMP induced by single agent Ixa was 23.5% and in Ibr-R cells 33%; increasing to 39% and 50.4%, respectively, after treatment with Ibr + Ixa. Mechanistic studies revealed changes in proteins regulated by the ubiquitin proteasome system and those associated with BTK signaling. While Ixa alone downregulated Bcl-2 and Mcl-1 levels, this effect was more marked in cells (both wildtype and Ibr-resistant) treated with Ixa + Ibr combination. Similar effects were noted in pPLCg2, albeit with some variability between the cell lines.
Conclusion: Using a combination of in silico modeling and experimental approaches, we provide first preclinical evidence on the antitumor effects of Ibr combined with the oral PI, Ixa, in WM cells, including those resistant to Ibr. Whereas further investigation on the precise molecular mechanisms of how Ibr + Ixa cooperate to induce apoptosis in WM cell is ongoing, our studies provide the basis for clinical testing of the Ibr + Ixa regimen; with a phase II clinical study in development.
Singh: Cellworks Research India Pvt. Ltd: Employment. Kumar: Cellworks: Employment. Basu: Cellworks: Employment. Trivedi: Cellworks: Employment. Majumdar: Cellworks: Employment. Abbasi: Cellworks Group Inc.: Employment. Vali: Cellworks Group Inc.: Employment. Martin: Janssen: Consultancy, Honoraria, Other: travel expenses; Genentech: Consultancy; Celgene: Consultancy; Teva: Research Funding; Novartis: Consultancy; Gilead: Consultancy, Other: travel expenses. Ansell: Bristol-Myers Squibb: Research Funding; Merck: Research Funding; Celldex: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding. Ailawadhi: Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pharmacyclics: Research Funding; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal